Inspection Readiness
FDA to Begin Unannounced Inspections of Non-U.S. Manufacturing Facilities
Beginning August 2025, FDA inspections at non-U.S. manufacturing sites will take place without advance notice. This shift eliminates prior warning and signals a new standard for global regulatory oversight—one that demands continuous readiness.
With deep FDA expertise and regulatory professionals positioned around the world, ProPharma helps pharmaceutical, biotechnology, and medical device companies build and sustain inspection readiness—before the FDA arrives.
FDA to Begin Unannounced Inspections of Non-U.S. Manufacturing Facilities
Beginning August 2025, FDA inspections at non-U.S. manufacturing sites will take place without advance notice. This shift eliminates prior warning and signals a new standard for global regulatory oversight—one that demands continuous readiness.
With deep FDA expertise and regulatory professionals positioned around the world, ProPharma helps pharmaceutical, biotechnology, and medical device companies build and sustain inspection readiness—before the FDA arrives.
Inspection Readiness is not an option – now, more than ever, it is urgent.
Our team of experts is equipped to guide you through every step of any inspection your firm is facing, with experience and expertise. The overview below outlines how ProPharma supports clients through every stage of the inspection readiness lifecycle.
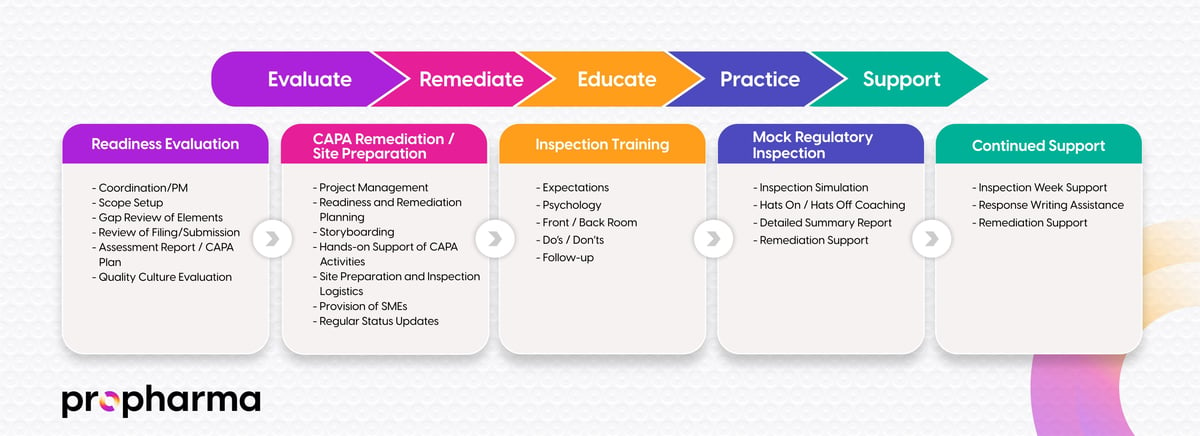
We ensure that every step towards Inspection Readiness is carefully calibrated to your needs. Our method is backed by tangible tools and training programs developed from the ground up, tailored not just to your product, but to your organization's unique scale and scope. This approach allows you to maintain focus on running your business, while we dedicate ourselves fully to your Inspection Readiness, making it our singular priority.
Inspection Readiness Experts
Unannounced inspections are coming. Preparation can't wait.
With global regulatory and quality expertise and a history of guiding clients through successful FDA inspections, ProPharma helps ensure your operations are always inspection ready—no matter when the FDA arrives.
Secure Your Regulatory Inspection Success
On average a new product takes approximately 10-15 years to develop, whether it is a biologic, a pharmaceutical product, a medical device, or a combination thereof.
- Can you risk the time and resources you invested in development by ignoring the importance of Inspection Readiness for a new product?
- Can you risk having an existing product off the market for remediation activities following a For Cause Inspection?
- Can your patients?
ProPharma's expertise covers all aspects of Inspections. Inspection Readiness Evaluation, Project Management, your Inspection Program/Process, Logistics, and Training – are just some of the most important areas of Inspection Readiness. Our expertise can become your greatest asset. We are eager to become your partner in navigating this critical process.


PAI Readiness
Pre-approval Inspections are one of the most critical inspections any Biologic, Pharmaceutical or Medical Device firm will undertake. Failure to meet FDA expectations can have a huge impact on organizations, including:
- Delays to product launch.
- Thousands, if not millions of dollars, in remediation costs.
- Lost market share.
- Loss of a firm's credibility.
PAI readiness is critical, and ProPharma is an industry leader in providing PAI Readiness to our clients.
BIMO Readiness
BIMO inspection readiness is essential for sponsors to ensure compliance with FDA regulations, protect data integrity, and uphold patient safety standards. By preparing thoroughly, sponsors:
- Ensure compliance with FDA regulations.
- Protect data integrity.
- Uphold patient safety standards.
- Help avoid costly delays.
- Prevent potential compliance issues.
- Facilitate smoother FDA inspections.

To date, we have had a 100% success rate on PAI Readiness projects for our clients
News & Insights

March 3, 2026
ANDA Development Made Clear: Expert Advice on Submission Strategy, Labeling, and...
Learn how strategic reference product selection, patent navigation, and labeling alignment under the 505(j) pathway drive efficient, approval-ready ANDA submissions.

March 2, 2026
Marketing Authorization and Market Access: Navigating Pricing & Reimbursement Be...
Explore the new EU HTA Regulation's impact on pricing and reimbursement, and learn key strategies for aligning market access with regulatory activities in Europe.

January 27, 2026
ProPharma Sets the Gold Standard in Sustainability with SBTi-Approved Net-Zero T...
ProPharma’s greenhouse gas reduction targets are validated by SBTi, aligning with the Net-Zero Standard and a commitment to reach net-zero by 2050.

December 11, 2025
ProPharma Expands Operations with New Office in Hyderabad
ProPharma expands with a new office in Hyderabad, enhancing innovation and growth in regulatory, clinical, and compliance services for the life sciences industry.

January 31, 2025
ProPharma Recognized for AI Excellence at ECCCSA
ProPharma wins Silver at ECCCSA for AI innovation in Medical Information, enhancing efficiency and quality in delivering accurate medical information.

October 9, 2024
ProPharma Receives 2024 CPHI Regulatory and Compliance Award
ProPharma wins CPHI Pharma Award for excellence in regulatory and compliance innovation, enhancing efficiency and accelerating market access for life-saving therapies.

February 5, 2026
Setting up a Global PV System
A company needed to rapidly establish a fully compliant global pharmacovigilance (PV) system, including UK/EU QPPV coverage, to meet regulatory requirements by a fixed deadline. With safety data and...

January 30, 2026
High-Volume Global Pharmacovigilance Onboarding
A global biotechnology sponsor faced a sudden surge in pharmacovigilance demand, with monthly ICSR volumes exceeding 30,000 cases across multiple regions. Limited internal capacity, tight onboarding...
.png?width=320&height=185&name=Webinar%20Thumbnail%20Quality%20%26%20Compliance%20(650%20x%20425%20px).png)
April 23, 2026
Clinical Promise to Commercial Reality: The Path to Cell & Gene Therapy Market
As cell and gene therapies transition from clinical development to commercialization, organizations face evolving regulatory expectations, expanded CMC requirements, and increased MAH...

April 9, 2026
EMA Policy 0070: Advanced Strategies for Compliance, Anonymization, and CCI Justification
As European Medicines Agency Policy 0070 enters its expanded Step 2 phase, sponsors face increased document volumes, heightened transparency obligations, and greater scrutiny of anonymization and CCI...
News & Insights

April 9, 2026
EMA Policy 0070: Advanced Strategies for Compliance, Anonymization, and CCI Justification
As European Medicines Agency Policy 0070 enters its expanded Step 2 phase, sponsors face increased document volumes, heightened transparency obligations, and greater scrutiny of anonymization and CCI...




