FDA
North America

June 30, 2022
Patient-Focused Drug Development: Selecting, Developing, or Modifying Fit-for-Purpose Clinical Outcome Assessments
Today, the U.S. Food and Drug Administration (FDA) issued a draft guidance, “Patient-Focused Drug Development: Selecting, Developing, or Modifying Fit-for-Purpose Clinical Outcome Assessments.” This...
ANDA
CDER
June 27, 2022
Providing Regulatory Submissions in Alternate Electronic Format Guidance for Industry
Final Guidance June 2022 This guidance provides recommendations on an alternate electronic format for submissions covered under an exemption from or a waiver of the requirements of section 745A(a) of...
ANDA
FDA

June 27, 2022
How to Interpret FDA’s Final Guidance - “Assessing the Effects of Food on Drugs in INDs and NDAs - Clinical Pharmacology Considerations”
On June 23, 2022, The FDA issued the final version of its Guidance for Industry titled “Assessing the Effects of Food on Drugs in INDs and NDAs - Clinical Pharmacology Considerations”. The most...
FDA
North America

June 24, 2022
FDA Issues Draft Guidance for Industry, Considerations for Rescinding Breakthrough Therapy Designation
Draft Guidance June 24, 2022 The U.S. Food and Drug Administration issued a draft guidance for industry titled Considerations for Rescinding Breakthrough Therapy Designation. This draft guidance...
EMA
Europe
June 24, 2022
EMA Procedural guidance for variant strain(s) update to vaccines intended for protection against Human coronavirus
8 June 2022 EMA/175959/2021 Rev.2 Human Medicines Division Regulatory and procedural requirements Introduction In order to ensure the continued effectiveness of authorised COVID-19 vaccines, it may...
FDA
North America
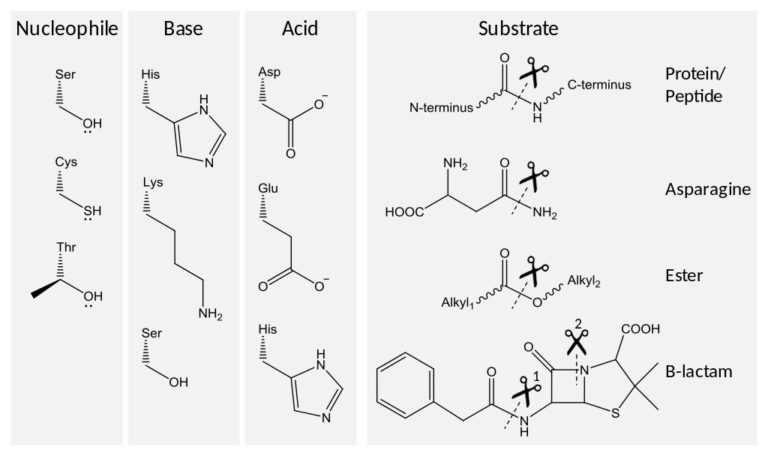
June 23, 2022
FDA revises 2013 guidance for Non-Penicillin Beta-Lactam Drugs
Draft Guidance June 2022 FDA is announcing the availability of a draft guidance titled, "Non-Penicillin Beta-Lactam Drugs: A CGMP Framework for Preventing Cross-Contamination." This guidance revises...
EMA
Europe

June 23, 2022
EMA Checklist for annual updates for parallel distribution - Draft Guidance
22/06/2022 EMA/405782/2020 Rev. 3 Human Medicines Division The European Medicines Agency (hereinafter 'the Agency') asks its applicants to use this checklist in advance of submission of an annual...
EMA
Europe
June 21, 2022
Advice on the designation of antimicrobials or groups of antimicrobials reserved for treatment of certain infections in humans - in relation to implementing measures under Article 37(5) of Regulation
Regulatory and Procedural Guideline May 2022 EMA/CVMP/678496/2021-rev Introduction “According to Article 37(5) of Regulation (EU) 2019/6 (‘the Regulation’), the European Commission shall adopt...
FDA
North America
June 21, 2022
Non-Clinical Performance Assessment of Tissue Containment Systems Used During Power Morcellation Procedures
June 2022 Draft Guidance for Industry and Food and Drug Administration Staff Introduction This draft guidance document provides recommendations that may help manufacturers comply with the special...









