June 2, 2020
June 2, 2020
In the first blog of this three-part series, “Overlooking Your QMS Could Cost You,” we discussed the cost of “good” versus “poor” quality, and the importance of investing in a “good” Quality Management System (QMS).
By spending your resources and time on prevention and improvement (“good” quality) you are ultimately focusing on mitigating risk. Risk that could have a devastating impact on your trial, drug, or device.
Risk, as defined by FDA’s Guidance on Quality Systems, is a continuum that requires constant attention.
The FDA concludes that modern quality systems, when coupled with manufacturing process, product knowledge, and effective risk management practices, can handle many types of changes to facilities, equipment, and processes without the need for prior approval regulatory submissions.
Manufacturers with a robust quality system and appropriate process knowledge, can implement many types of improvements. In addition, an effective quality system that lowers the risk of manufacturing problems may result in shorter and fewer FDA and EMA inspections.
When it comes to clinical trials and clinical laboratories, the same risk philosophies have been adopted from the device/drug manufacturing industries. The industry defines risk as any event that may have an undesired impact on patient safety, compliance, or data integrity. Risk mitigation is the process of developing options, actions, and reactions (e.g., quality system documents, investigation documents, training, CAPA, and protocol amendments) to reduce threats to the success of the trial, analytical testing, or conformance of the device or drug to specifications.
A QMS developed with a risk-based focus can help mitigate risk from the beginning of the drug development or device design process and provide a foundation to address issues as they are encountered.
Quality system design establishes a means to maintain compliance and oversight by creating an infrastructure for Good Manufacturing Practice (GMP) and Good Clinical Practice (GCP) QMS.
Essentially, your QMS is the embodiment of compliance from beginning to end. A strong Quality System has many components that are critical for mitigating risk and should accomplish the following:
A proactive and risk-based approach to a quality system can substantially reduce the chances of your organization falling victim to a catastrophic risk that could jeopardize your trial, drug, or device’s future.
With a robust Quality System in place, you will be able to successfully bring your drug or device to market, while providing a safe and effective treatment for those who need it.
ProPharma’s Life Science Consulting team is made up of subject matter experts who can assist with Clinical and Commercial Quality System designs including risk management, quality manuals, SOPs, CAPA design and processes, risk management plans, and communication plans. We also assist with Quality System implementation and optimization, as needed. Additionally, we can conduct qualifying audits for sites and vendors, routine audits for ongoing trial, CMO, and CRO audits.
Whether your needs are for implementing, maintaining, or remediating a Quality Management System, our risk mitigation experts are here to help. Contact us today for a complete evaluation of your current QMS.
Stay tuned for Part 3 of this blog series, where we will discuss common QMS obstacles and how you can avoid them.
TAGS: Life Science Consulting
June 17, 2021
In July 2018, FDA issued a draft guidance document entitled “Testing of Retroviral Vector-Based Human Gene Therapy Products for Replication Competent Retrovirus During Product Manufacture and Patient...
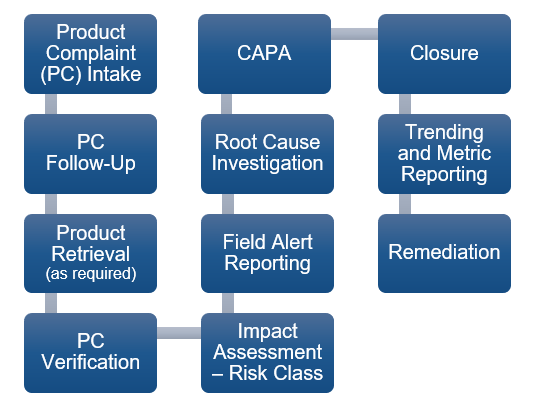
March 10, 2021
The Product Quality Complaint program is an essential tool in a company’s quality and compliance toolkit, not only for reducing patient risk and enhancing customer satisfaction, but because it...
May 19, 2021
Gene therapy holds the promise of curing severe genetic diseases at the genetic level rather than merely treating the symptoms as is accomplished using conventional small-molecule drug therapies. In...