August 17, 2016
August 17, 2016
During product development some sponsors struggle to determine how their product will be regulated by the FDA. Will it be considered a drug, medical device, biologic, or combination product? Based on the product’s primary mode of action, the FDA makes determines how the product will be classified and which offices will carry out the premarket review. This determination can have a significant impact on a product’s development; as such, FDA allows sponsors to request a designation early by submitting a Request for Designation (RFD).
On August 11th, the FDA announced that it will be making some changes to its “internal procedures for responding to communications from sponsors regarding preliminary product classification assessments from OCP.” Among these “changes” described by the FDA is the introduction of the Pre-Request for Designation (Pre-RFD) process, which will allow sponsors to seek a product classification from FDA, but requires less information than a formal RFD submission.
According to FDA’s blog post, the Pre-RFD process can be used at any point during product development. For sponsors looking to use a more interactive approach to engage the Agency, the Pre-RFD process may be preferable to the more formal RFD process. FDA states that sponsors may find the Pre-RFD process beneficial for the following reasons:
While there are a number of benefits to the Pre-RFD process, there are also many similarities between the RFD and Pre-RFD processes. These include:
FDA notes that its feedback is based on the information that is submitted, thus “sponsors should bear in mind that the speed and quality of any review, whether Pre-RFD or formal RFD, is highly dependent on the quality of the submitted data.”
In addition, FDA also states that it is in the process of developing a draft guidance on the Pre-RFD process, and that it plans to publish a list of product classifications for various types of products. The Agency welcomes any and all feedback concerning the RFD and Pre-RFD processes, and encourages individuals to voice any questions, comments, or concerns that they may have.
Are you in the process of developing a combination product? We can help you obtain a designation for your product and achieve a successful interaction with FDA. To learn more about our services and how we can help you, contact us today.
February 21, 2012
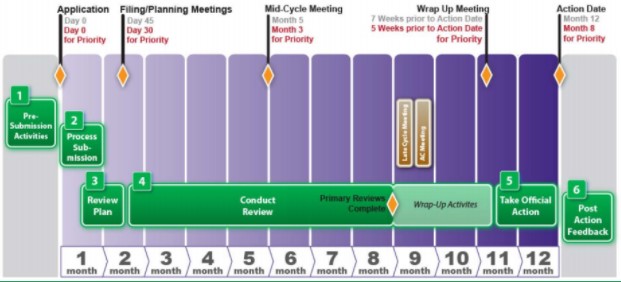
Compiling and submitting an NDA is a complicated and intensive activity. Once you have submitted your application to FDA, you may be curious about what can you expect to hear and when. Below are some...
February 8, 2017
On Monday, February 6th, the FDA released the new process that will be used to collect and post curricula vitae (CVs) for advisory committee members. This will help FDA to post the appropriate CVs to...
February 27, 2017
On Wednesday, February 15th, Senators Michael Bennet (D – CO) and Johnny Isakson (R – GA) introduced a bill to improve FDA’s medical device inspection process. If passed, the bill would amend the...