March 29, 2022

March 29, 2022

In order to incentivize the development of therapies (drugs biologics) to fill unmet medical needs for treatment of serious conditions, the FDA has developed various programs to expedite drug development and review. These four programs are: fast track, breakthrough therapy, accelerated approval, and priority review. Over the years, we have learned that guiding clients through the process and selecting the right expedited program can have great benefits. Understanding which track best suits each product substantially aids in creating an effective regulatory strategy and drug development program.
Before discussing the FDA’s expedited programs in detail, it is useful to review some definitions:
There is often confusion regarding the FDA’s expedited programs, as they have many similarities and overlapping benefits. All four programs are designed to address an unmet medical need for a serious condition.
Here, we summarize the procedural guidance from the FDA regarding these expedited programs, with an emphasis on comparing and contrasting the four programs. The table below and following information provides an overview of all four of FDA’s expedited programs.
| Type of Data Required | Data Should Demonstrate | Benefits | |
|---|---|---|---|
| Fast Track Designation | Preliminary nonclinical, mechanistic, or clinical data | Potential to address an unmet medical need for a serious condition |
|
| Breakthrough Therapy Designation | Preliminary clinical data | Substantial improvement on clinically significant endpoint(s) over available therapies |
|
| Accelerated Approval Pathway | Not specified; Sponsor should make justification of alternate endpoint based scientific support | Generally provides a meaningful advantage over available therapies AND demonstrates an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit or a clinical endpoint that can be measured earlier than irreversible morbidity or mortality |
|
| Priority Review Designation | Data contained in the final NDA submission | Significant improvement in safety or effectiveness of the treatment, prevention, or diagnosis of a serious condition |
|
For more information, we’ve also provided an in-depth breakdown of each expedited program below. Furthermore, we also have a video presentation "Expedited Programs Explained: How to Expedite Product Approval in the US and Europe" and a blog post explaining European expedited regulatory programs.
The Fast Track Designation process helps to facilitate the development and expedite the review of new drugs that treat a serious medical condition and fill an unmet medical need. By speeding up these processes, new drugs can get to patients in need faster than they normally would through standard tracks.
Fast Track Designation is intended to address unmet medical needs and a wide range of serious conditions. A drug may be granted Fast Track Designation if it is believed to have an impact on patient survival, day-to-day functioning, or if it is believed that the condition will progress in severity if left untreated. Examples of serious conditions that treatment drugs may receive Fast Track Designation for include: AIDS, Alzheimer’s, heart failure, cancer, epilepsy, depression, and diabetes.
If a therapy already exists, Fast Track Designation may still be granted if it is believed a therapy will serve an unmet medical need by potentially being better than available therapies.
Drugs that receive Fast Track Designation from the FDA are eligible for some or all of the following benefits:
Drug companies can request Fast Track Designation, and the request can occur any time during the drug development process. Upon receipt, the FDA will review the Fast Track Designation request and make a decision within 60 days. If granted, it is encouraged for drug companies to have frequent communication with the FDA to ensure questions and issues are resolved quickly so the drug can be approved soon and get to patients who would benefit from it.
Breakthrough Therapy Designation from the FDA helps expedite the development and review of new drugs that may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. A clinically significant endpoint refers to an endpoint that measures an effect on irreversible morbidity or mortality (IMM), or on symptoms that are serious consequences of the disease.
The determination of whether the improvement over available therapy is substantial and depends on the magnitude of treatment effect including the effect duration and observed clinical outcome. Typically, to obtain Breakthrough Therapy Designation the preliminary clinical evidence should clearly indicate an advantage over available therapy.
Drugs that receive Breakthrough Therapy Designation from the FDA are eligible for some or all of the following benefits:
Drug companies can request Breakthrough Therapy Designation, and the FDA may recommend doing so if they believe the drug development program meets the criteria and if the program would benefit from the designation. The FDA recommends that the Breakthrough Therapy Designation request should occur before the end-of-phase-2 meetings to ensure drug companies can receive the benefits of the designation.
Fast Track and Breakthrough Therapy are the most similar programs designed to expedite the development of drugs for serious conditions. The most significant difference in these two programs is related to the type of data needed to substantiate the request. Fast Track Designation can be granted based on preliminary data, such as activity in a nonclinical model or pharmacological data, or a mechanistic rationale. Breakthrough Therapy Designation must use preliminary clinical data, and therefore activity in a nonclinical model or a mechanistic rationale alone would not be sufficient.
Sponsors should also note the subtle differences in the designation criteria: drugs seeking Fast Track Designation must only have the potential to address an unmet medical need, while drugs seeking Breakthrough Therapy Designation must have preliminary data which demonstrate substantial improvement on clinically significant endpoints over available therapies.
The benefits of Fast Track Designation are more frequent meetings with FDA, more frequent written communication from the Agency, and rolling review. Rolling review refers to the ability of FDA to begin review of the NDA as sections are completed, rather than waiting for complete submission of the entire NDA to begin review. Breakthrough Therapy Designation drugs receive all the benefits of Fast Track drugs, but also are given intensive guidance on an efficient drug development program and have the involvement of FDA senior managers. It is not uncommon for a drug with Fast Track Designation to be granted Breakthrough Therapy Designation during the drug development process.
Accelerated Approval is the most unique of the expedited programs for drugs intended to treat a serious disease because it is an approval pathway rather than a designation. An approval pathway is a mechanism to market authorization whereas a designation is granted to a drug based on meeting certain criteria. A designation provides certain benefits, such as expediting the approval process (for priority review designations) or providing tax credits and exclusivity (for orphan drug designations).
Accelerated approval allows for the use of a surrogate endpoint including a laboratory measurement, radiographic image, physical sign, or other measure that is reasonably likely to predict clinical benefit. It also allows for the use of an intermediate clinical endpoint that is considered reasonably likely to predict the clinical benefit of a drug, such as an effect on irreversible morbidity or mortality. Using surrogate or intermediate clinical endpoints saves time in the drug approval process, but Sponsors will need to provide sufficient scientific support for the FDA to approve the proposed surrogate or intermediate clinical endpoint. Drugs using the accelerated approval pathway must also generally provide a meaningful advantage over available therapies.
Accelerated approval has an important commitment; the Sponsor is required to conduct additional clinical trials after the drug is approved to confirm the clinical benefit. Until the benefit has been confirmed in a post-market setting, the labeling has special language to indicate that the use of the drug has not yet been shown to have a clinical benefit. Additionally, if a drug fails to show a clinical benefit after approval, the drug can be removed from the market.
The final FDA expedited program is Priority Review, which directs attention and resources to evaluating drugs that are believed to potentially provide significant improvements in the safety or effectiveness of the treatment, diagnosis, or prevention of serious conditions over standard applications.
While most NDAs have a 10-month goal date, priority review drugs have a 6 month goal date which benefit a Sponsor by significantly expedite the application process.
Priority Review is requested by a Sponsor at the time of NDA submission. In order to qualify for priority review, the Sponsor must show that if approved, the drug would provide:
The FDA will inform Sponsors of their decision to grant Priority Review Designation within 60 days of the receipt of the original application.
If you think your drug may be eligible for one or any of the FDA’s expedited programs, have additional questions about one or all of the programs, contact ProPharma Group. ProPharma Group is the global leader in regulatory science consulting services with over 35 years of experience helping companies of every size across the world achieve success with the FDA.
We’ll use our extensive knowledge and experience working directly with the FDA to facilitate a successful interaction with the Agency. Contact us to learn more about our services and get started today.
November 1, 2018
On Monday, October 12th, FDA issued a draft guidance regarding the role of Pre-IND Meetings in the development of drugs to treat rare diseases. The document, entitled "Rare Diseases: Early Drug...
October 25, 2022
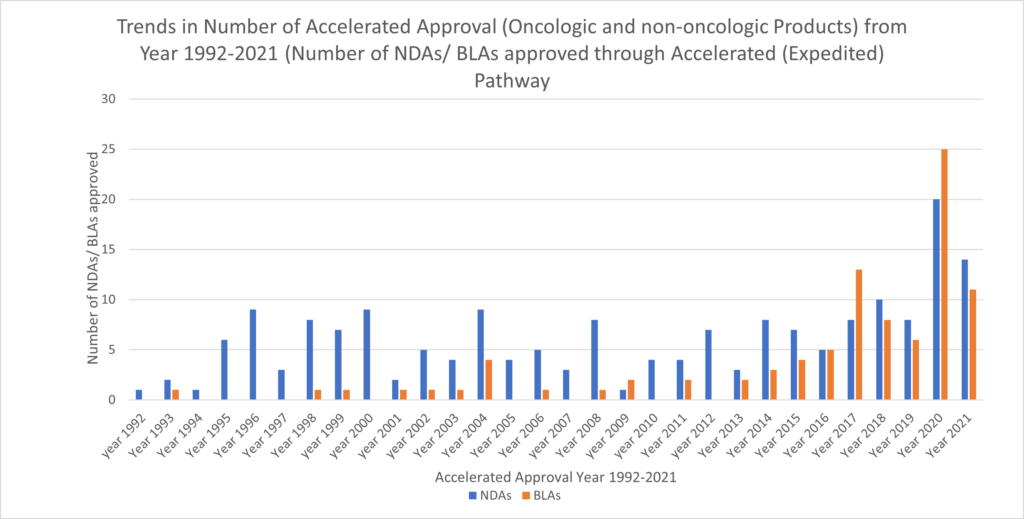
The accelerated approval provisions of FDASIA in section 506(c) of the FD&C Act provide that FDA may grant accelerated approval to: . . . a product for a serious or life-threatening disease or...
July 18, 2016
On Thursday, July 7th, the FDA issued two draft guidance documents regarding the compounding of drugs that are essentially copies of commercially available or approved drugs. In these documents,...