January 10, 2018
January 10, 2018
At the beginning of each federal fiscal year, the US FDA posts the previous year’s Form 483 observation metrics issued by each product center.
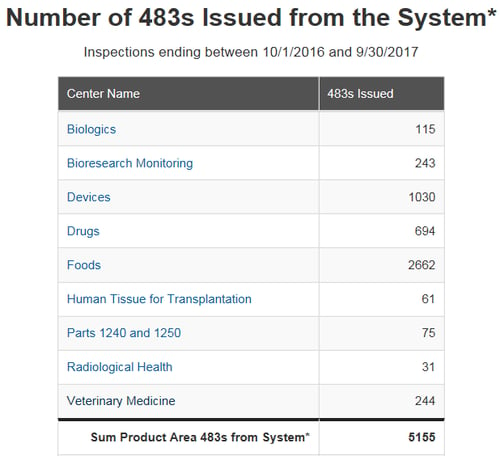
I find that reviewing these metrics provides a valuable snapshot of industry’s compliance direction. Understanding these common issues can help highlight focal areas when evaluating potential quality system gaps and improvement opportunities within your company.
Summaries of the top five observation occurrences per sector are as follows:
The 2017 top 5 observations reported by the Drug sector are:
The 2017 top 5 observations reported by the Devices sector are:
The 2017 top 5 observations reported by the Biologics sector are:
The 2017 top 5 observations reported by the Veterinary sector are:
Over 2,000 observations were issued by FDA’s Office of Regulatory Affairs in 2017 across the four FDA regulated industries listed above, Drug, Device, Biologics, and Veterinary. What do these numbers imply, what can we learn from this data, is your site at risk for one of these observations? As an industry what, can be done to help avoid receiving one of these common observations?
While the regulations haven’t changed drastically in recent years, the compliance expectation is ever increasing. FDA utilizes computerized systems (e.g., Turbo 483) that allow inspections to be more risk-based and better focus inspections on industry trends, guiding inspectors towards common noncompliance areas within industry quality systems. The trend shows not following procedures, inadequate record handling, and inadequate investigations are frequent themes in industry.
As an industry, we should be prepared for FDA’s increased focus on these recurring themes. I find that a good starting point for risk reduction is understanding industry’s trouble areas. One way to approach this is by taking a closer look at the governing quality systems that cover the top ranking observations. For instance, review the thoroughness of your site’s training program and/or simplify complex or confusing SOPs to help avoid a “procedures not followed” observation. Preventing observations relating to “inadequate investigations” look to implemented / trained upon Root Cause Analysis procedures for possible gaps. Monitoring the status of your site’s quality systems and reacting to reported quality metrics helps to avoid these common observations and facilitates continuous improvements.
I like to keep common industry pitfalls in mind when evaluating the capability and key quality indicators of a quality system, this helps me determine whether a state of control is maintained throughout product lifecycle, and gauge how effectively the facility manages risks. This practice helps me direct the quality system towards improvement and increased compliance.
When reflecting on last year’s top issues, thoughts of the famous quote by George Santayana are evoked, “Those who cannot remember the past are condemned to repeat it.”
May 30, 2019
At the beginning of each federal fiscal year, the US FDA posts the previous year's Form 483 observation metrics issued by each product center. Inspections ending between 10/1/2017 and 9/30/2018, for...
April 25, 2017
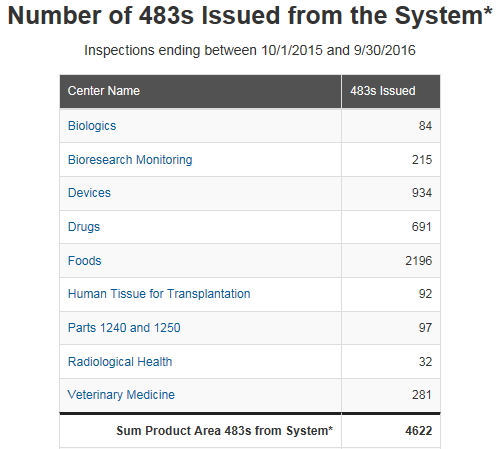
At the beginning of each year, the US FDA posts the previous year’s Form 483 observation metrics issued by each product center. Image Source: FDA I find that reviewing these metrics provides a...

May 18, 2022
The FDA’s Office of Clinical Pharmacology within the Office of Translational Sciences released a new draft guidance document that, for the first time, clearly addresses the FDA’s recommendations and...