April 22, 2014

April 22, 2014


It is amazing to me that more C-Level Executives do not perceive their Quality Systems as a powerful vehicle to reduce costs, improve productivity, and benefit their bottom line. Rather than embrace and require the operation of their quality systems with this perspective in mind, they frequently regard cGMP compliance (and therefore internal Quality personnel and functions) as a necessary government enforced evil, a non-profitable cost center, and a barrier to optimized business speed and nimbleness. Organizations run by executives with this perspective are likely to have weak quality systems that may achieve minimal regulatory compliance, but will never achieve the true benefits of a quality system designed to continually improve itself thereby reducing waste and costs. There is no shortage of recalls, warning letters, consent decrees and even criminal prosecutions as examples of companies with marginal quality systems.
Why is this? There are several answers of course, but one path to understanding is recognition and appreciation of the corporate executives that the organization looks to for guidance; the very individuals responsible for an organization’s culture, leadership, and ultimately the Quality System itself.
Executives, for better or worse, live in a slightly different world than most folks and, although they are humans, they are still a peculiar bunch. So much so that they even speak a different language: the language of strategic design and direction (or “Executive-ese”). Strategic design and direction, after all, is an executive’s “raison d'etre” andleads an organization down a critical path that determines its very existence; its ability to profit, grow, sustain, grow some more, and accomplish the objectives set forth in its mission statement. Also, make no mistake about it: executives are really, really focused on the bottom-line.
Executive-ese is detail adverse and only focuses on primary business drivers rather than any attempt to understand lower-level details associated with strategic business initiatives (details are for underlings…that’s why executives hire them).
Furthermore, Executive-ese is populated with catch-phrases including "improve customer satisfaction", "increase market share", "decrease operating expenses", "increase revenue", "beat the competition", "shorten time to market", "improve employee performance" and "reduce turn-over", among others.
The opportunity for the QA professional is adapting quality related communications and data in a manner that the C-level executive will understand. For example, if you can propose a quality initiative of interest in a high level way that clearly presents real and tangible contributions to the bottom line, then you are speaking “executive-ese”. Consequently, your chances of being taking seriously and obtaining your desired outcome are exponentially raised. Executives are not dumb by any means and can recognize a “no-brainer” when they see one.
First and foremost, you must understand that a Quality System is an extremely powerful way to accomplish the catch-phrase objectives listed above, all of which if realized, contribute favorably to the bottom line. There is a reason though that quality system excellence is not used more strategically in the bio-medical industries and that’s because many C-level executives do not appreciate the difference between compliance and quality.
Compliance is simply putting out sufficient effort to meet cGMP’s minimum requirements and measuring compliance against the law. Quality, on the other hand, is derived from a systematic continuous improvement process whereby management, resources, products, and measurement systems are aligned and operated in such a way that continuous improvement (and compliance) is an inescapable outcome. Look at ISO13495 and ICH Q10 as continuous improvement quality system models.
So, how do you enlighten C-Level executives on their misconceptions as well as affect enough change whereby the quality system becomes a driving force for process cost savings, productivity improvements, and waste reduction contributions to the bottom line? One approach is executive-ese laced quality metric data.
Tips for speaking Executive-ese:
Examples:
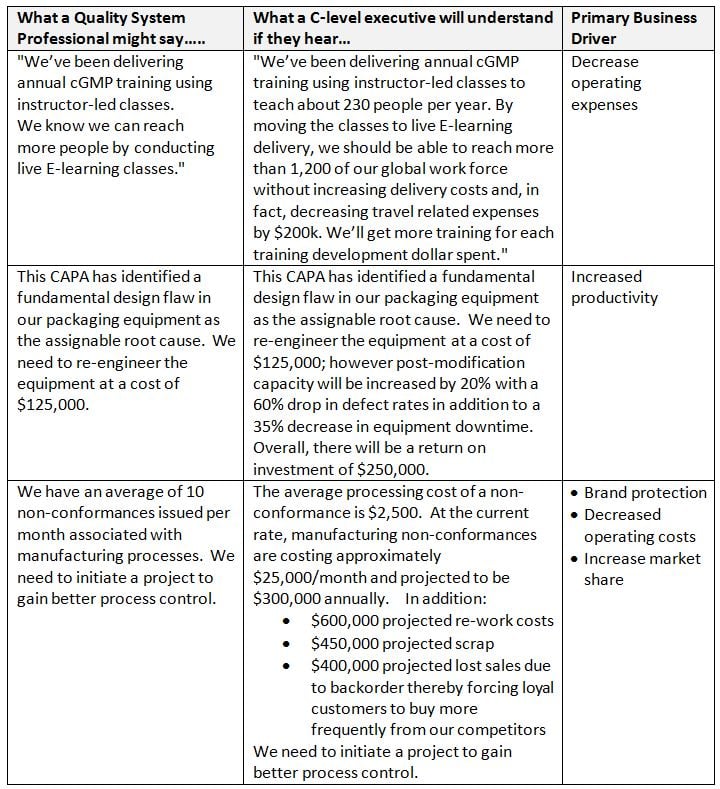
Also:
Learn more about ProPharma Group's Compliance services.
Contact us to get in touch with our subject matter experts for a customized Compliance presentation.

June 20, 2013
On May 24, 2013, the Department of Justice announced that ISTA Pharmaceuticals Inc., a subsidiary of Bausch+Lomb, pled guilty to felony charges of conspiracy to introduce a misbranded drug into...

March 12, 2014
At the recent Pharmaceutical Compliance Congress (PCC), Zane Memeger, U.S. Attorney for the Eastern District of Pennsylvania stated that one of the key questions that a company should ask about its...